How does something get shipped out without first having been approved to do so at a large manufacturing company? Who knows, but this is what apparently recently happed at Merck & Co Inc.
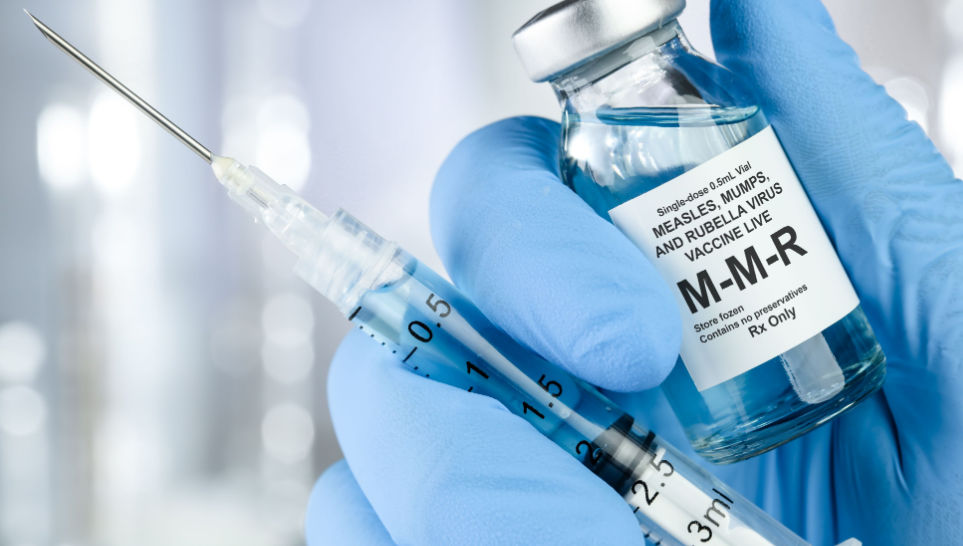
As per the Food and Drug Administration website, Merck & Co Inc. is voluntarily recalling a batch of vaccines for measles, mumps, and rubella after it accidentally shipped doses to its U.S. customers before internal company approval for market release, U.S. health regulators said.
The lot affected is Lot # 0851AA of M-M-R® II, which contained approximately 39,000 doses of a vaccine, due to the inadvertent shipment of doses from the lot to US customers. These doses were distributed by Merck between May 17, 2012 and May 25, 2012, prior to final internal approval for market release.
The company, based in Whitehouse Station, N.J., said it was recalling the batch as a precaution and already has asked customers to return the 39,000 vaccines.
An investigation by Merck concluded that there were no product safety, quality, or efficacy concerns associated with the batch, the U.S. Food and Drug Administration said on its website.
Merck does not expect this to have any impact to the supply of the vaccine as they distribute about 10 million doses of MMRII in the U.S. every year. It said the recall shouldn’t affect its ability to meet this demand.
Merck has contacted customers directly to provide instructions on how to return the product, the statement said. In addition, customers who have purchased product from the affected lot from a vaccine distributor or wholesaler will be provided instructions from their respective distributor or wholesaler regarding the return of product in their possession.
This is the only information posted on the FDA website concerning this information. For additional information, please contact the Merck National Service Center at 1-800-672-3672 (Monday to Friday 8 AM to 7 PM Eastern Time).
I know accidents happen, but come on! I am so glad that someone figured this out before they were administered!