The Flucelvax Vaccine, officially the Flucelvax Quadrivalent, is a cell-based flu vaccine first approved in 2016. Flucelvax is an inactivated vaccine designed for seasonal use.
While, according to the CDC, Flucelvax and similar cell-culture vaccines could offer higher safety than traditional egg-based vaccines, some individuals may still suffer allergic reactions and other adverse effects to Flucelvax. Let’s recap the basics about Flucelvax, how it works, and its possible adverse effects.
What Is the Flucelvax Vaccine?
The Flucelvax vaccine is an influenza vaccine by CSL Seqirus. The process of its creation involves dog kidney cells, compared to chicken eggs in traditional variations of the flu shot. The vaccine grows in an artificial cell culture, and its manufacturing process doesn’t harm animals.
The Flucelvax vaccine production starts with the CDC providing the vaccine manufacturer with a candidate vaccine virus or CVV. The Flucelvax quadrivalent influenza vaccine includes four CVVs. The next step is the inoculation of the cultured cells with the CVVs. After the CVVs replicate, the manufacturer collects the virus-containing culture, purifies the antigens, and prepares the vaccine.
How Does the Flucelvax Vaccine Work, and What Is Its Composition?
The active ingredient in Flucelvax is the influenza virus haemagglutinin, a glycoprotein that allows the virus to bind to cell surface receptors and enter the cytoplasm. When a person receives a Flucelvax shot, their immune system recognizes these glycoproteins as foreign elements and develops antibodies that defend the vaccinated person whenever they encounter the same virus in its active form.
Other ingredients in Flucelvax include the mercury derivative thimerosal (a preservative) and residual amounts of cell proteins and DNA, polysorbate, cetyltrimethylammonium bromide, and propiolactone from the manufacturing process.
Common and Severe Side Effects Associated With Flucelvax Vaccine
The CDC estimates that in a typical year, flu vaccines prevent millions of medical visits and save thousands of lives. On a nationwide scale, the benefits of Flucelvax and other influenza vaccines outweigh the risks, and receiving an annual flu shot is an almost universal recommendation. However, a very small percentage of people will experience serious side effects after the flu vaccination.
Like other flu vaccines, Flucelvax may result in adverse effects ranging from common (like mild injection site pain and transient flu-like symptoms) to rare and extremely severe. Let’s list a few possible serious side effects of the Flucelvax vaccine.
Allergic and Anaphylactic Reactions
Various components in flu vaccines may cause severe allergic reactions in certain individuals. One prominent and serious condition is anaphylaxis, an acute hyper-reaction of the immune system that can cause a sudden blood pressure drop, breathing difficulties, fainting, and potentially lethal blockage of air passages.
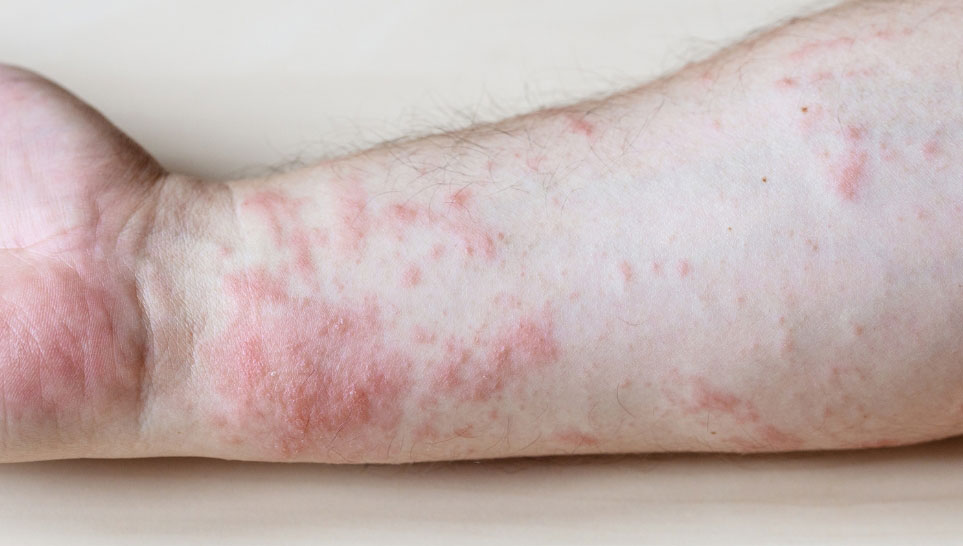
Erythema Multiforme
Erythema Multiforme is a severe allergic skin reaction that may sometimes occur after flu vaccines. Characteristic symptoms include raised, red lesions all over the body, but especially on the face, hands, and feet. The condition usually resolves with anti-inflammatory medications.
Guillain-Barrè Syndrome
Guillain-Barrè Syndrome (GBS) is a very rare and violent neurological disorder that could occur post-vaccination. In GBS, the immune system attacks the peripheral nervous system. GBS symptoms include sensory changes, weakness, coordination issues, and sometimes paralysis. Treatment of GBS includes a stage of acute care and a prolonged rehabilitation period.
Paresthesia
Paresthesia is a persistent prickling or burning “pins and needles” sensation, usually in the extremities, face, or back. Paresthesia symptoms may subside after a few days or last for months. Treating paresthesia usually focuses on resolving the underlying cause, but treatment options are limited for people who suffer from vaccine-induced paresthesia.
SIRVA

Shoulder Injury Related to Vaccine Administration, or SIRVA, is a preventable side effect of receiving the Flucelvax vaccine. SIRVA happens when the medical practitioner injects the vaccine dose into the shoulder joint instead of the muscle. Possible effects include shoulder inflammation and reduced range of motion.
Dizziness and Fainting
Some people may experience dizziness or fainting after a Flucelvax vaccine because of needle anxiety or for other reasons. The main danger of fainting is a fall that could cause serious injuries, especially in seniors.
Tonic-Clonic Seizures
Tonic-clonic seizures, or seizures with limb stiffening followed by jerking, and twitching movements, often occur in epilepsy because of abnormal neural activity in the brain. Very rarely, this type of seizure may also happen after vaccination, even without a prior history of seizures.
Angioedema
Angioedema, or sudden, extreme swelling, may occur after a vaccine in some individuals. The main danger of angioedema is swelling of the throat or tongue, which can block airflow and cause suffocation. Angioedema treatment may require medications like antihistamines or steroids.
Drug Interactions
Flucelvax could potentially interact with some immunosuppressive therapies like irradiation, alkylating agents, corticosteroids, and antimetabolites. Individuals who take blood thinners are at an increased risk of bleeding after a Flucelvax shot. You should inform your healthcare provider of all your medical conditions and prescription drugs before receiving a Flucelvax vaccine.
It’s important to remember that the aforementioned side effects are rare, and the majority of people will experience only mild after-effects following a Flucelvax vaccine. However, when adverse effects do occur, they are often unpredictable and severe. If you experience serious side effects after a Flucelvax dose, you have the right to seek legal recourse and potentially collect compensation for vaccine injuries.
What Are the Benefits of the Flucelvax Vaccine?

Flucelvax protects recipients against four A and B subtype influenza strains, chosen annually based on the prevalent strains in that year’s flu season. A Flucelvax shot can protect against influenza and reduce the severity of symptoms if you do become sick. For the elderly, children, and immunocompromised people, the Flucelvax vaccine can protect against flu complications, hospitalizations, and even death.
Differences Between the Flucelvax Vaccine and Other Flu Vaccines
Unlike traditional flu vaccines, the manufacturing process of the Flucelvax vaccine doesn’t require any chicken eggs. Egg-free vaccines offer numerous benefits, the first of which is their higher suitability for people with egg allergies.
Flucelvax is also faster to produce thanks to the cell culture method, which would make this vaccine a better option for mass vaccination during a flu pandemic. Flucelvax Quadrivalent is currently the only flu vaccine in the U.S. that uses cell cultures to grow vaccine viruses.
Where Can the Flucelvax Vaccine Be Obtained?
It’s possible to receive the Flucelvax vaccine in licensed clinics and health care centers across the U.S. You can search for influenza vaccines near your location by zip code on Vaccines.gov.
Availability and Cost of the Vaccine
Flucelvax Quadrivalent is available in pre-filled syringes with a single dose (0.5 ml) for injecting into the muscle.
Health insurers typically cover Flucelvax and other annual flu shots as preventive care. This is true for private insurers, Medicaid, Medicare, and people who enjoy coverage via the Affordable Care Act. Otherwise, the cost of a Flucelvax shot may range from $35 to $45, depending on the provider. You can check with your local pharmacy for Flucelvax prices.
Are There Any Age Restrictions and Contraindications?

The FDA currently approves Flucelvax starting at six months of age. People with a history of severe allergic reactions to any component of Flucelvax shouldn’t receive this vaccine.
Other possible contraindications to Flucelvax include:
- A history of another flu vaccine triggering Guillain-Barrè Syndrome within six weeks of the injection
- Acute fever (the health care provider should delay vaccine administration until the fever ceases)
- Major bleeding disorders
If you have any misgivings about receiving the Flucelvax vaccine, discuss your concerns with your healthcare provider.
Who Should Receive the Vaccine?
With rare exceptions, the CDC recommends Flucelvax for all people six months of age or older. Flu vaccines are especially important in populations that are more susceptible to influenza complications, like children and seniors.
Adults and children over nine years old who receive Flucelvax will usually require one dose per year. Children younger than nine who hadn’t received the shot before may require a second booster dose four weeks after receiving the first dose.
Is the Flucelvax Vaccine Safe for Pregnant Women?
So far, no randomized clinical trials assessed the safety of the Flucelvax vaccine during pregnancy. However, collected data from pregnant women who received a Flucelvax dose hasn’t revealed any adverse pregnancy outcomes that would be attributable to this vaccine.
The ACOG (American College of Obstetricians and Gynecologists) recommends pregnant women receive a flu shot at any stage of pregnancy to protect themselves from possible complications of getting sick.
Limited knowledge is available on the effect of Flucelvax on breastfeeding mothers. It’s unclear whether Flucelvax can pass to babies through human milk. However, flu antibodies pass through milk, so nursing mothers who receive a Flucelvax shot may boost immunity in their infants.
Did You Suffer an Adverse Effect of Flucelvax? Contact Sadaka Law
Do you believe you suffered an allergic reaction, a severe autoimmune response, or another vaccine injury because of the Flucelvax vaccine? Call Sadaka Law. Our experienced vaccine injury lawyers have won millions for clients over our 15+ years of handling vaccine injury cases nationwide.
Call 800-810-3457 or contact us online for a case evaluation. Learn more today.